Antimony »
PDB 1exi-8rtm »
2w0h »
Antimony in PDB 2w0h: X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph
Enzymatic activity of X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph
All present enzymatic activity of X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph:
1.8.1.12;
1.8.1.12;
Protein crystallography data
The structure of X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph, PDB code: 2w0h
was solved by
P.Baiocco,
G.Colotti,
S.Franceschini,
A.Ilari,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 103.14 / 3.00 |
| Space group | P 41 |
| Cell size a, b, c (Å), α, β, γ (°) | 102.945, 102.945, 193.129, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 23.8 / 26.2 |
Antimony Binding Sites:
The binding sites of Antimony atom in the X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph
(pdb code 2w0h). This binding sites where shown within
5.0 Angstroms radius around Antimony atom.
In total 2 binding sites of Antimony where determined in the X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph, PDB code: 2w0h:
Jump to Antimony binding site number: 1; 2;
In total 2 binding sites of Antimony where determined in the X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph, PDB code: 2w0h:
Jump to Antimony binding site number: 1; 2;
Antimony binding site 1 out of 2 in 2w0h
Go back to
Antimony binding site 1 out
of 2 in the X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph
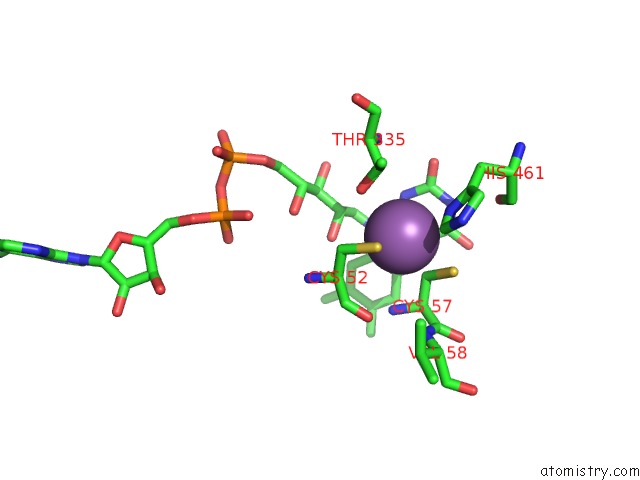
Mono view
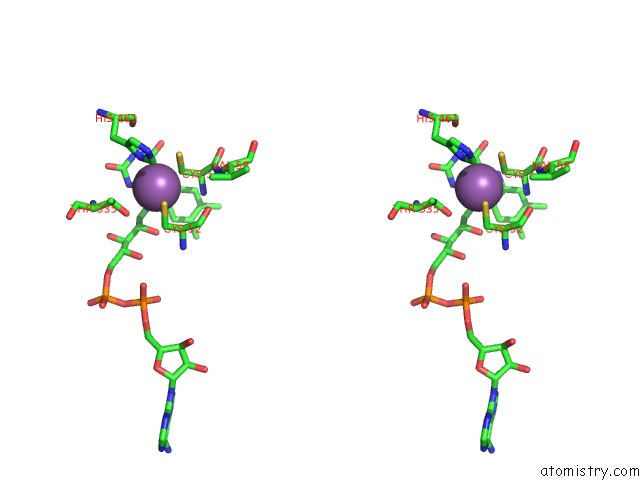
Stereo pair view

Mono view

Stereo pair view
A full contact list of Antimony with other atoms in the Sb binding
site number 1 of X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph within 5.0Å range:
|
Antimony binding site 2 out of 2 in 2w0h
Go back to
Antimony binding site 2 out
of 2 in the X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph
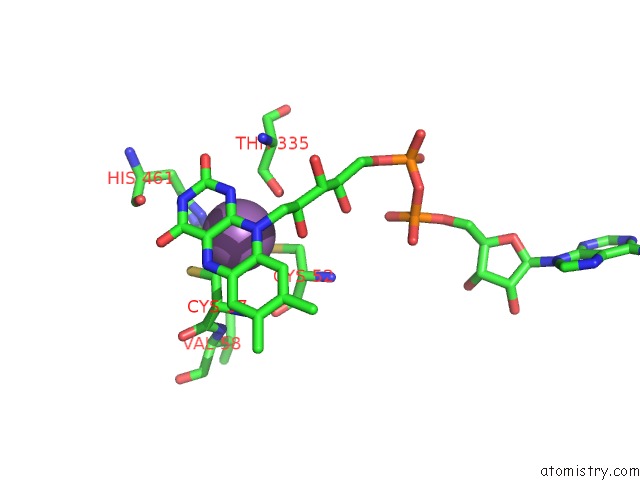
Mono view
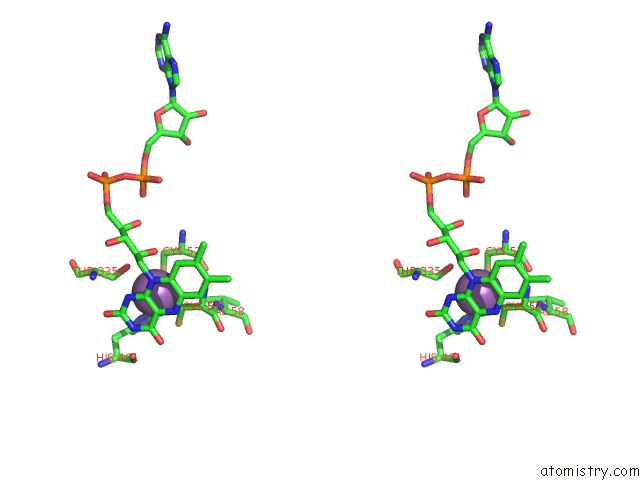
Stereo pair view

Mono view

Stereo pair view
A full contact list of Antimony with other atoms in the Sb binding
site number 2 of X Ray Structure of Leishmania Infantum Trypanothione Reductase in Complex with Antimony and Nadph within 5.0Å range:
|
Reference:
P.Baiocco,
G.Colotti,
S.Franceschini,
A.Ilari.
Molecular Basis of Antimony Treatment in Leishmaniasis. J.Med.Chem. V. 52 2603 2009.
ISSN: ISSN 0022-2623
PubMed: 19317451
DOI: 10.1021/JM900185Q
Page generated: Tue Aug 19 01:15:11 2025
ISSN: ISSN 0022-2623
PubMed: 19317451
DOI: 10.1021/JM900185Q
Last articles
Sr in 3E4PSr in 3CCU
Sr in 3CCV
Sr in 3CD6
Sr in 3CCS
Sr in 3CCR
Sr in 3CCQ
Sr in 3CCM
Sr in 3CCL
Sr in 3CCJ